Abstract
Background: Naratuximab emtansine (nara, Debio 1562, formerly IMGN529) is an antibody-drug conjugate (ADC) consisting of a humanized anti-CD37 antibody, K7153A, conjugated via a thioether-based linker to a cytotoxic maytansinoid, DM1. CD37, a lymphocyte surface marker, is highly expressed in B-NHL. A Phase 1 monotherapy study of nara revealed a good safety profile and promising efficacy (22% overall response rate [ORR] in DLBCL at all doses). In preclinical models of B-NHL, nara demonstrated synergistic antitumor activity when combined with rituximab (RTX).
Aim: The aim of thisopen-label Phase 2 study was to evaluate the safety and efficacy of nara + RTX and to characterize pharmacokinetics (PK) and pharmacodynamics (PDy) in patients (pts) with relapsed and/or refractory (R/R) B-NHL.
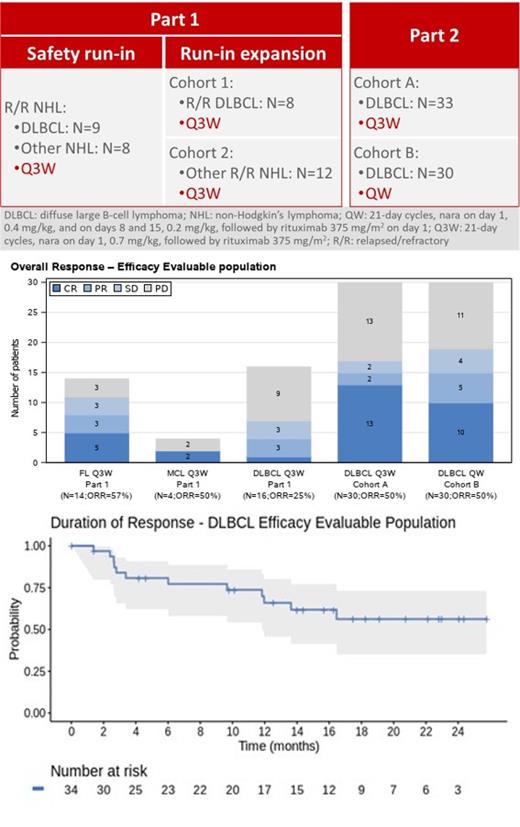
Methods: R/R B-NHL pts who were not candidates for stem cell transplant, with 1-6 prior lines of treatment, were recruited to two study parts. In Part 1, which included a safety run-in followed by an expansion, pts received 0.7 mg/kg nara in combination with 375 mg/m 2 RTX every 3 weeks (Q3W). In Part 2, only R/R DLBCL pts were included. Pts were assigned either to the Q3W regimen (cohort A), or to a weekly regimen of 0.4, 0.2, and 0.2 mg/kg nara administered on days 1, 8 and 15 of 21-day cycles combined with 375 mg/m 2 RTX on day 1 (cohort B). Six cycles of treatment were administered with possible extension. Primary endpoints were safety and ORR. PK and PDy evaluations included ADC and DM1 catabolites' systemic levels and receptor occupancy on peripheral blood mononuclear cells (PBMCs), to investigate CD37 target engagement. Pts from cohorts A and B were requested to fill in the FACTLym quality of life questionnaire (QoL). Pts with double/triple hit lymphoma, bulky disease, and up to 6 prior lines of treatment for DLBCL were allowed. There was no limit on life expectancy. Pts were considered efficacy evaluable (EE) if they had one baseline and at least one post-baseline tumor assessment or an assessment of clinical progression. Tumor assessment by CT was acceptable. The follow-up period was up to 1 year after last pt first dose (NCT02564744).
Results: 100 pts were enrolled in the study: 80 DLBCL, 14 follicular lymphoma (FL) and 6 mantle cell lymphoma (MCL) pts; 81 pts (81%) experienced grade ≥ 3 treatment emergent adverse events, the most common being neutropenia 54 (54%), leukopenia 19 (19%), lymphopenia 17 (17%), and thrombocytopenia 12 (12%). Of the 80 DLBCL pts, 10 (12.5%) were primary refractory, 24 (30%) were refractory to last line, 62 (78%) had Ann Arbor stage III/IV, and 35 (44%) had received at least 2 prior systemic cancer therapies. The ORR in the 76 EE DLBCL pts was 44.7%, with 24 (31.6%) complete responses (CR) and 10 (13.2%) partial responses (PR). In addition, 9 (11.8%) stable disease (SD) and 33 (43.4%) progressive disease (PD) were observed. 30 pts were efficacy evaluable in each of the two major cohorts (A and B) of Part 2, enrolling mainly relapsed pts. ORR was 50% in each cohort (CR rate: 43.3% in cohort A; 33.3% in cohort B). In Part 1, 16 DLBCL pts were EE, of which 6 were primary refractory and 10 were refractory to last line. ORR in this group was 25%. Median duration of response (mDoR) in the 76 evaluable DLBCL pts was not reached (lower 95% confidence interval [CI] 12 months). Median duration of follow-up in responders was 15 months (95% CI 9-18 months). In the 14 FL pts, the ORR was 57%: 5 CR, 3 PR, 3 SD, and 3 PD; the mDoR in this population was not reached (lower 95% CI: 19 months), with a median duration of follow-up of 21.8 months (95% CI 19.1 - not reached). Of the 6 MCL pts, 4 were efficacy evaluable: 2 CR and 2 PD. Due to the low number of pts, the DoR curves are not presented. PK levels were sufficient to fully engage the CD37 target on PBMCs. A relationship between PK exposure and efficacy was identified. Data showed acceptable systemic release of cytotoxic DM1 and catabolites. DLBCL responders in cohorts A and B demonstrated on average a clinically meaningful improvement of 3 points (standard deviation: 6.6) in the Lymphoma Subscale of the FACT-Lym QoL.
Summary/Conclusion: The combination of nara + RTX resulted in good OR and CR rates, durable responses, a manageable safety profile, and full CD37 target engagement. Consequently, nara + RTX could be considered an attractive option for the treatment of R/R B-NHL. The treatment was well tolerated and contributed to the pts' well-being, as demonstrated by the QoL results.
Levy: Janssen Pharmaceuticals: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; GSK: Consultancy, Other: Promotional speaker; Karyopharm: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Amgen Inc.: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Gilead Sciences, Inc.: Consultancy, Honoraria, Speakers Bureau; Beigene: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Other: Promotional speaker; Morphosys: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Epizyme: Consultancy, Other: Promotional speaker; Dova: Consultancy, Other: Promotional speaker; TG Therapeutics: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau. Grudeva-Popova: University Hospital St George: Current Employment; Roche: Speakers Bureau; Abbvie: Speakers Bureau; Amgen: Speakers Bureau; Novartis: Speakers Bureau; Takeda: Speakers Bureau. Trněný: Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; MorphoSys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Celgene: Consultancy; 1st Faculty of Medicine, Charles University, General Hospital in Prague: Current Employment; Portola: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Amgen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses. Jurczak: Loxo Oncology: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Debbiopharm: Research Funding; Celgene: Research Funding; Celtrion: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; Sandoz: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Epizyme: Research Funding; Incyte: Research Funding; Merck: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding. Pylypenko: Communal nonprofit enterprise "Cherkasy regional oncology dispensary of Cherkasy oblast council: Current Employment. André: Johnson & Johnson: Research Funding; Gilead: Consultancy, Other: Travel/Accommodations/Expenses; Incyte: Consultancy; Roche: Other: Travel/accomodation/expenses, Research Funding; Celgene: Other: Travel/accomodation/expenses; AbbVie: Other: Travel/accomodation/expenses; Karyopharm: Consultancy; Bristol-Myers-Squibb: Consultancy, Other: Travel/Accommodations/Expenses; Takeda: Consultancy, Research Funding. Dwivedy Nasta: Roche: Research Funding; Pharmacyclics: Research Funding; Millenium: Research Funding; ATARA: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Merck: Other: Data safety monitoring board; Rafael: Research Funding; Debiopharm: Research Funding. Rechavi-Robinson: Debiopharm International SA: Current Employment. Toffanin: Debiopharm International SA: Current Employment. Micallef: F. Hoffmann La Roche: Ended employment in the past 24 months; Debiopharm International SA: Current Employment. Attinger: Debiopharm International SA: Current Employment. Rouits: Debiopharm International SA: Current Employment, Patents & Royalties. Roubaudi-Fraschini: Debiopharm International SA: Current Employment. Orfanos: Debiopharm International SA: Current Employment; F. Hoffmann La Roche: Ended employment in the past 24 months. Dymkowska: CANTARGIA AB: Consultancy; AUREALIS OY: Consultancy; Debiopharm International SA: Consultancy; Morphosys AG: Consultancy. Nauwelaerts: Novartis Pharmaceuticals AG, Basel: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Debiopharm International SA: Current Employment. Woei-a-Jin: University Hospitals Leuven, Belgium: Current Employment; Recordati: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Kyowa Kirin: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal